Carbon Capture Technologies
In my previous post on “Management of Carbon Emissions in Industries”, I have briefly mentioned about carbon capture and storage. In this post, I am going discuss various technologies used for carbon capture.
3. Oxy-Fuel Combustion Capture
Carbon dioxide has been captured from industries for more than 80 years. It is captured for industrial uses such as urea and ammonia production, carbonated beverage production, breweries. Capture technologies have three categories, depending on what stage of the process carbon dioxide is removed. These are:-
- Pre-Combustion Capture
- Post Combustion Capture
- Oxy-Fuel Combustion Capture
 |
| Figure 1: Capture Technologies |
1. Pre-Combustion Capture
Here, carbon dioxide is separated from the fuel before combustion. It is done at very high temperature by splitting hydrocarbons into hydrogen and carbon dioxide. The carbon dioxide is removed before combustion and the carbon dioxide undergoes combustion to form water. The hydrocarbons can be split by processes like gasification of coal and reforming of natural gas.
In natural gas reforming process, natural gas, water vapour and air react together in a reactor to form carbon monoxide and hydrogen. Reforming takes place at high temperature and pressure. The synthesis gas further reacts with water in a water gas shift reactor to form carbon dioxide and hydrogen. The carbon dioxide is separated from the product stream using amine absorption and hydrogen is combusted to produce water vapour. Nowadays, majority of hydrogen produced is diverted to hydrogen fuel cells. The carbon dioxide is then captured for storage purposes.
In coal gasification process, coal is reacted with steam at pressure below 100 bar and temperature above 750 degree Celsius to form synthesis gas (mixture of carbon monoxide and hydrogen) and small amount of carbon dioxide and methane depending on process conditions. After gasification stage, carbon dioxide is separated out from other gases.
In coal gasification process, coal is reacted with steam at pressure below 100 bar and temperature above 750 degree Celsius to form synthesis gas (mixture of carbon monoxide and hydrogen) and small amount of carbon dioxide and methane depending on process conditions. After gasification stage, carbon dioxide is separated out from other gases.
Capturing carbon dioxide before combustion offers some advantages. First, carbon dioxide is not diluted by other constituents of combustion air. Second, the carbon dioxide containing stream is usually at high temperature.
 |
| Figure 2 : Pre-combustion capture |
2. Post Combustion Capture
This is the most common method for separating carbon dioxide from gas stream. Here, carbon dioxide is separated from the flue gas after combustion. This type of capture method can be fitted into many types of emitters in both power plants and industrial plants. Separation equipment can be fitted on existing emission sources. Carbon dioxide can be separated from flue gas by using amines and ammonia.
i. Capturing Carbon Dioxide by using Amine
In this process the flue gas flows through an absorption tower or scrubber. The flue gas comes in contact with amine (usually monoethanolamine) mixed with water which binds with carbon dioxide in a relatively weak carbon bond. The bound carbon dioxide is transported to another tower known as stripping tower where solvent is heated which separates carbon dioxide and amine in a process known as regeneration. The amine is then reused to absorb more carbon dioxide.
The flue gas which is usually at about 80 degree Celsius is cooled to 40 degree Celsius before absorption.
ii. Capturing Carbon Dioxide by using Ammonia
The process of using ammonia for capturing carbon dioxide is similar to that of amine. The main advantage of using ammonia is that the regeneration requires less energy. The problem with ammonia is that it is highly volatile and evaporates easily. Therefore, the flue gas needs to be cooled to 10 degree Celsius before separation. The chilled ammonia is used to separate carbon dioxide from flue gas.
The flue gas is cooled to a temperature between 0 to 10 degree Celsius. The cooled flue gas is transported to the absorber where it meets ammonia in aqueous solution. The carbon dioxide reacts with ammonia to produce ammonium carbonate and ammonium bicarbonate. The remaining flue gas is led through a washing process to remove any remaining ammonia. The solution is sent to the desorber where it is heated and carbon dioxide and ammonia are separated.
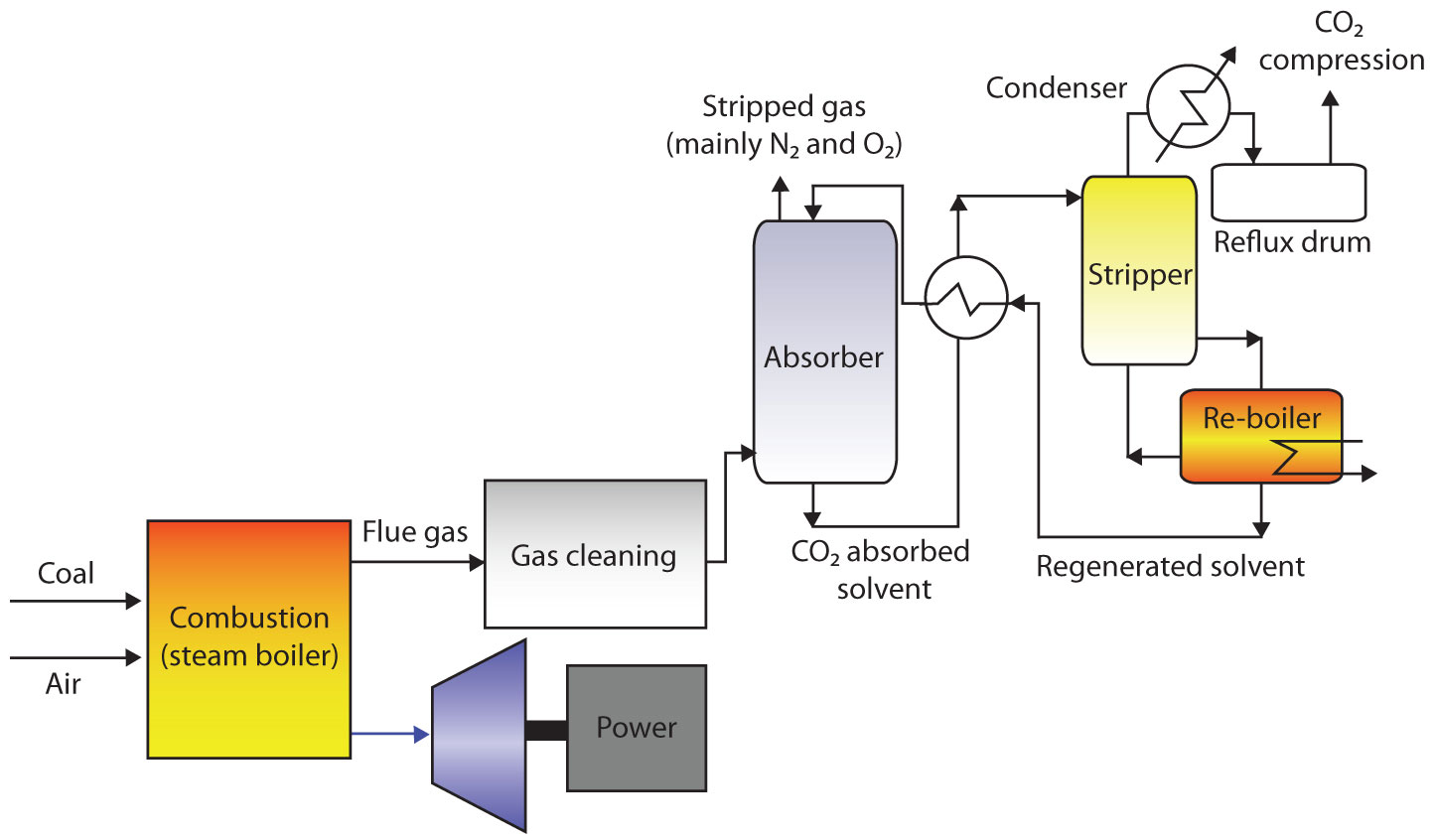 |
| Figure 3: Post Combustion Capture |
3. Oxy-Fuel Combustion Capture

Figure 4 : Oxy-fuel combustion Capture

In this process, combustion takes place using pure oxygen instead of air. The exhaust gas consists of carbon dioxide and water vapour. The flue gas is cooled so that water condenses into liquid and gets separated from carbon dioxide. Combustion with pure oxygen generates very high temperatures. This process simplifies carbon dioxide capture as no nitrogen is involved. This process can be competitive with post combustion capture process but the experience with this process is limited.
References:
References:

Comments
Post a Comment